Introduction
Degeneration and liquefaction of the vitreous gel with age ultimately leads to formation of a posterior vitreous detachment (PVD), characterized by the separation of the posterior cortical vitreous from the internal limiting membrane. While PVD represents a normal process of the aging eye, incomplete separation or focal adhesions of the vitreous gel to the macula may result in abnormalities of the vitreoretinal interface.
Advances in ophthalmic imaging have focused our understanding of disorders involving the vitreomacular interface. In 2013, the International Vitreomacular Traction Study (IVTS) Group introduced an optical coherence tomography (OCT) based system for the classification of vitreomacular interface disease. Specifically, the terms vitreomacular adhesion (VMA), vitreomacular traction (VMT), and full-thickness macular hole (FTMH) were defined. FTMH was defined as a foveal lesion involving all retinal layers. VMA was defined as macular attachment of the vitreous cortex within a 3-mm radius of the fovea without change in retinal morphology. VMT was differentiated from VMA by the presence of retinal morphologic changes, but without FTMH (Figure). Both VMA and VMT could be further classified by the size of adhesion [focal (≤1500 μm) or diffuse (>1500 μm)] as well as the presence or absence of concurrent macular disease. FTMH was classified as primary (due to VMT) or secondary and further defined by size as small (≤250 μm), medium (>250 and ≤400 μm), or large (>400 μm).
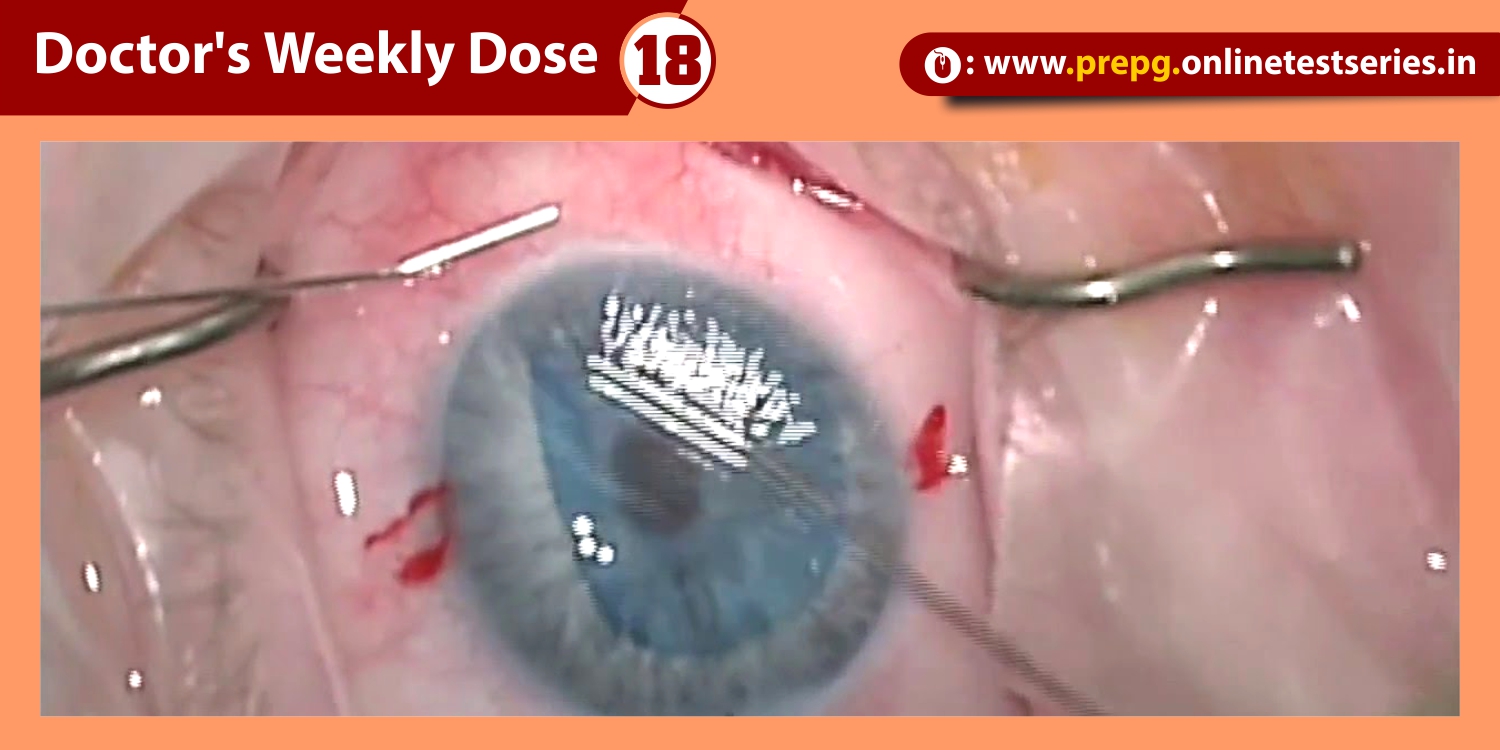
Resulting abnormalities in retinal morphology from VMT commonly lead to visual decline, including metamorphopsia, visual field defect, and decreased visual acuity . Surgical management with pars plana vitrectomy (PPV) has long been the mainstay of VMT and FTMH treatment . However, following the U.S. Food and Drug Administration approval of intravitreal ocriplasmin (Jetrea®; ThromboGenics, Inc., Iselin, NJ, USA) for the treatment of VMT, pharmacologic vitreolysis is now a viable therapeutic option. Ocriplasmin, a 27 kilodalton serine protease, achieves its effect via lysis of laminin and fibronectin at the vitreoretinal interface and subsequent VMA release.
Pharmacologic vitreolysis with ocriplasmin, a 27 kilodalton serine protease, is an effective nonsurgical treatment option for vitreomacular traction (VMT). Data from phase III clinical studies, including the Microplasmin for Intravitreal Injection—Traction Release without Surgical Treatment (MIVI-TRUST) and Ocriplasmin for Treatment for Symptomatic Adhesion Including Macular Hole (OASIS) studies, have demonstrated the treatment efficacy of ocriplasmin for VMT and full-thickness macular hole (FTMH). Subgroup analysis of these clinical trials as well as post-marketing clinical series have aided in patient selection by identifying features associated with successful pharmacologic release of VMT with ocriplasmin, including adhesion diameter ≤1500 μm, absence of depiretinal membrane, phakic status, and age younger than 65. As a first-in-class therapeutic, ocriplasmin and its side effects have been carefully monitored by the vitreoretinal community. The following categories of related or possibly related adverse events have been identified: acute reduction in visual acuity, ERG changes, dyschromatopsia, retinal tear or detachment, lens subluxation or phacodonesis, abnormal pupillary reflex, retinal vascular changes, and OCT ellipsoid zone alterations. Adverse events have almost all been transient with restoration of visual acuity; however, in select patients, alterations may persist.
Conclusion
Data from Phase III Clinical Trials and post-marketing studies have expanded our understanding of pharmacologic vitreolysis with ocriplasmin. Ocriplasmin has been demonstrated as an effective nonsurgical therapy for VMT release and FTMH closure, with patients on average experiencing improvement in best-corrected visual acuity and visual function. Importantly, careful patient selection improves VMT release success rates. Ocriplasmin has been carefully monitored for adverse events, and these, particularly acute but almost entirely self-limited visual disturbances, are important for patient consent and understanding. Structural changes on OCT are common and often related to successful VMT release, the etiology of which remains incompletely understood. Overall, adverse events have been largely transient, with restoration of visual acuity; however, in select patients, alterations may persist. Surgical outcomes in eyes with a prior history of ocriplasmin treatment have been favorable and comparable to control eyes.
Future areas of interest include the efficacy of ocriplasmin for VMT in patients with concurrent macular disease and the role of ocriplasmin for other vitreoretinal surgery indications, including pediatric retinal detachment and diabetic tractional detachment. Additionally, trials directly comparing outcomes with pneumatic vitreolysis (intraocular perfluropropane gas or sulfur hexafluoride gas) would be of special interest in determining the relative efficacy of ocriplasmin compared to treatment alternatives. Prospective, phase IV studies in addition to improved post-marketing reporting of adverse events will be helpful in refining the ongoing risk/benefit profile of ocriplasmin use.