Acute renal failure (ARF), characterized by sudden loss of the ability of the kidneys to excrete wastes, concentrate urine, conserve electrolytes, and maintain fluid balance, is a frequent clinical problem, particularly in the intensive care unit, where it is associated with a mortality of between 50% and 80%.
Diagnostic evaluation of ARF
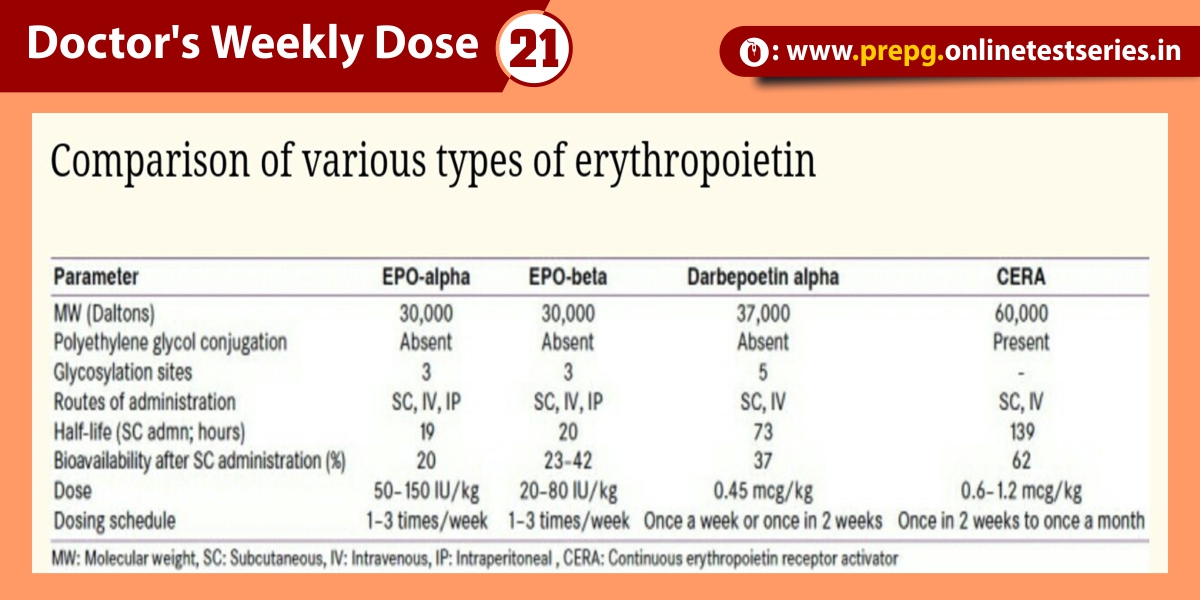
It was well established that if tubular function was intact, renal vasoconstriction was associated with enhanced tubular sodium reabsorption. Specifically, the fraction of filtered sodium that is rapidly reabsorbed by normal tubules of the vasoconstricted kidney is greater than 99%. Thus, when nitrogenous wastes, such as creatinine and urea, accumulate in the blood due to a fall in glomerular filtration rate (GFR) secondary to renal vasoconstriction with intact tubular function, the fractional excretion of filtered sodium (FENa = [(urine sodium × plasma creatinine) / (plasma sodium × urine creatinine)]) is less than 1%. An exception to this physiological response of the normal kidney to vasoconstriction is when the patient is receiving a diuretic, including mannitol, or has glucosuria, which decreases tubular sodium reabsorption and increases FENa. It has recently been shown in the presence of diuretics that a rate of fractional excretion of urea (FEurea) of less than 35 indicates intact tubular function, thus favoring renal vasoconstriction rather than established ARF as a cause of the azotemia. Also, renal vasoconstriction in a patient with advanced chronic renal failure may not be expected to be associated with an FENa of less than 1 because of chronic adaptation to an increased single-nephron GFR. Specifically, the adaptive decrease in tubular reabsorption to maintain sodium balance in chronic renal disease may make the interpretation of FENa difficult in this setting.
The approximately 80% diagnostic specificity of FENa in distinguishing azotemia associated with renal vasoconstriction and intact tubular function from established ARF with tubular dysfunction may result from limited sensitivity of this parameter, or, perhaps more likely, the patient may actually be progressing from a prerenal azotemic state to established ARF. With established ARF the urine-concentrating capacity is abolished; thus measurement of urinary osmolality may complement the use of FENa in the diagnostic separation of renal vasoconstriction from established ARF in the patient with a rising BUN measurement and serum creatinine level.
Given image shows guidelines for urinary indices whereby established ARF can be distinguished from renal vasoconstriction with intact tubular function, i.e., prerenal azotemia. It also should be pointed out that some causes of ARF, including radiocontrast media and myoglobinuria, may be associated with an FENa of less than 1
Mechanisms of ARF
The mechanisms of ARF involve both vascular and tubular factors . An ischemic insult to the kidney will in general be the cause of the ARF . While a decrease in renal blood flow with diminished oxygen and substrate delivery to the tubule cells is an important ischemic factor, it must be remembered that a relative increase in oxygen demand by the tubule is also a factor in renal ischemia.
Renal vascular abnormalities
Loss of autoregulation and increased renal vasoconstriction: the role of increased cytosolic and mitochondrial calcium.
Outer medullary congestion
Outer medullary congestion of the kidney is another vascular hallmark of acute renal ischemia. This congestion has been proposed to worsen the relative hypoxia in the outer medulla and thus the hypoxic injury in the S3 segment of the proximal tubule and the thick ascending limb of the Henle loop
Tubular abnormalities
There is no question that renal tubule dysfunction occurs in established ARF, since tubular sodium reabsorption is decreased, i.e., FENa > 2.0, at a time when normal renal tubules avidly increase tubular sodium reabsorption in response to renal vasoconstriction.
Structural changes during ischemic ARF
ARF is characterized by tubular dysfunction with impaired sodium and water reabsorption and is associated with the shedding and excretion of proximal tubule brush border membranes and epithelial tubule cells into the urine.
Translocation of Na+/K+-ATPase from the basolateral membrane to the cytoplasm could explain the decrease in tubular sodium reabsorption that occurs with ARF.
Tubular obstruction in ischemic ARF
The existence of proteolytic pathways involving cysteine proteases, namely calpain and caspases, may therefore explain the decrease in proximal tubule sodium reabsorption and increased FENasecondary to proteolytic uncoupling of Na+/K+-ATPase from its basolateral membrane anchoring proteins.
Tubuloglomerular balance and tubular fluid backleak in ischemic ARF
The decrease in proximal tubular sodium reabsorption that is associated with acute ischemic injury would increase sodium chloride delivery to the macula densa and thereby activate the tubuloglomerular feedback mechanism and decrease GFR
Therapies
Renal replacement therapy for ARF generally involves intermittent hemodialysis (IHD) or continuous renal replacement therapy (CRRT), e.g., continuous veno-veno-hemofiltration (CVVH).
Prolonged duration of the ARF clinical course and the need for dialysis are major factors projecting a poor prognosis. Patients with ARF who require dialysis have a 50–70% mortality rate. Infection and cardiopulmonary complications are the major causes of death in patients with ARF.